What Are Some of the Uses of Propylene Glycol?
By on Nov 5th 2020
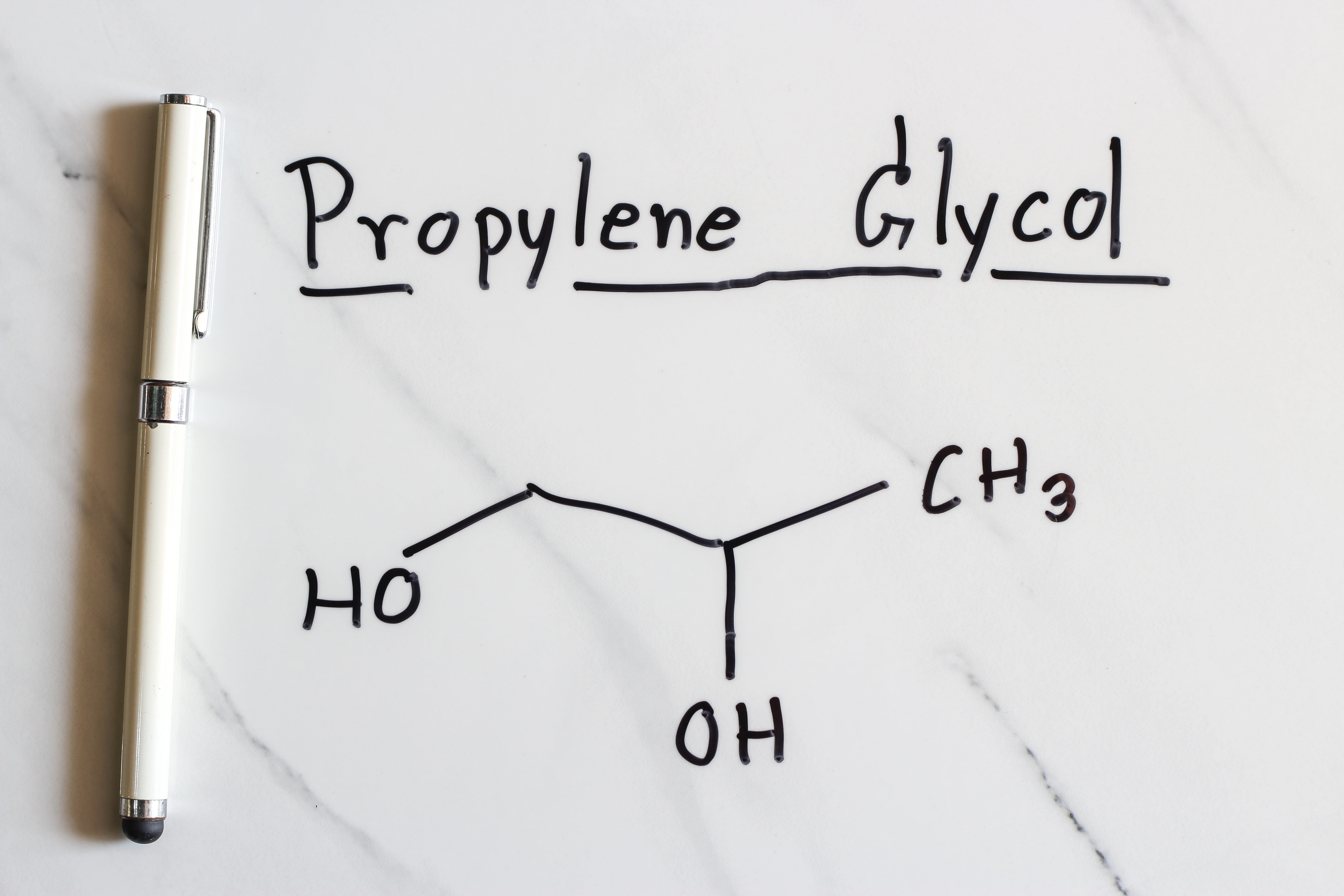
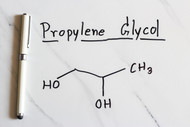
Propylene Glycol (PG) is a food additive that is typically made two similar ways industrially. One way PG can be made is by heating food-grade propylene oxide, which comes from petroleum to 200-220°C. The other way that PG is made is very similar and involves the same propylene oxide and a small amount of an acid promoter to be heated to 150-180°C. This is a simple and safe industrial synthesis. The word “Glycol” simply means that the compound belongs to the alcohol family and has two alcohol functional groups on the molecule or two “-OH” groups. The word “Propylene” on the other hand states the number of carbons in the molecule, in this case three. Propylene Glycol is comprised of 3 carbon atoms and 2 alcohol groups.
How Is Propylene Glycol Used in Food?
PG is commonly used in many different foods including ice-cream, beverages, and different types of bread. Propylene Glycol is used because of its ability to hold moister and its sweet taste. PG is non-irritating, has a low volatility and it also acts as an emulsifier. An emulsifier allows two different substances that wouldn’t normally mix to stay in a homozygous mixture, or where they are uniform and not separating.
But how do these qualities create better ice-cream? Having low volatility means that propylene glycol doesn’t evaporate as easily as other liquids that might be more volatile such as water. PG also may act as a carrier for flavors, fragrances, vitamins, and food dyes. All of these qualities are extremely important when trying to produce the best ice-cream as well as beverages and other baked goods. When baking bread, it helps retains moisture and reduces the production time. Additionally, it is widely used in cosmetics for these same reasons.
Other Uses for Propylene Glycol

Propylene
Glycol is also considered a “Humectant” which is why it is commonly used in
toothpaste. A humectant basically will absorb water and continue to stay moist.
This is helpful in toothpaste to maintain moisture and prevent it from drying
out.

All of these performance qualities that make PG useful in food and cosmetics also allow it to be a useful ingredient in other things like Automotive Antifreeze/Coolant. Propylene glycol is used to make non-toxic, and environmentally friendly antifreeze but often leads to confusion with its cousin ethylene glycol.
Propylene Glycol vs. Ethylene Glycol
Propylene glycol is very different from ethylene glycol. Propylene glycol is generally used as a food additive and is generally recognized as safe (GRAS) by the FDA 21 C.F.R. Propylene Glycol can be added to food and has no side effects according to the FDA. Ethylene glycol though similar in properties is not nearly as safe. Ethylene glycol is not permitted for use in food or cosmetic items, that is why it is primarily used in automotive antifreeze where its similar properties can be useful. If Ethylene glycol is ingested symptoms can include vomiting and seizures.
What Is the Fog in a Fog Machine?

Another cool use of propylene glycol is in heated fog machines. These fog machines use a mixture normally called “Fog Juice” this fog juice generally consists of food-grade PG with varying amounts of distilled water. These fog machines are also used by firefighters for drills. PG in mixture with other chemicals can also be pumped through vents in some hospitals to help disinfect them. It is also used in inhalers and vapor products. Overall, propylene glycol is very versatile and has a lot of uses.
At
Ingredi.com, we sell Propylene Glycol USP in 3 different sizes pails, drums or totes. Tell us what you use this product for and you can save 5%!
Dow Propylene Glycol USP | 43 lbs Pail
Dow Propylene Glycol USP | 480 lbs Drum
Dow Propylene Glycol USP | 2300 lbs Tote
Sources:
https://www.healthline.com/nutrition/propylene-glycol#TOC_TITLE_HDR_3
https://foodadditives.net/solvent/propylene-glycol/
https://www.atsdr.cdc.gov/phs/phs.asp?id=1120&tid=240.
https://biobalancenow.com/why-fogging-is-the-best-way-to-combat-systemic-mold-in-your-home/
https://pubmed.ncbi.nlm.nih.gov/23656560/
https://whs.rocklinusd.org/documents/Science/Lethal_Dose_Table.pdf
https://www.who.int/news-room/fact-sheets/detail/salt-reduction.
https://www.britannica.com/science/glycol
https://www.cdc.gov/niosh/ershdb/emergencyresponsecard_29750031.html